BRIEF INTRODUCTION:
Commodity Name: Dextrose monohydrate
CAS No.: 5996-10-1
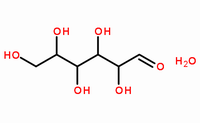
Molecular Formula: C6H12O6·H2O
Molecular Weight: 198.17
Molecular Structure:
Description: Dextrose monohydrate is produced from corn starch. Dual enzymatic conversion technology has been successfully applied to our production in the starch into glucose. By concentration, crystallization, dried, filtered and evaporated to a series of processes, the raw materials converted to a white crystalline hexagonal flakes, i.e. oral glucose. Oral glucose has been widely applied to food and beverage products, as a substitute sweetener. After endotoxin dispose, can get injection grade dextrose monohydrate. Dextrose monohydrate is a white, crystalline powder, with a sweet taste, freely soluble in water, sparingly soluble in alcohol. The natural dextrose exists widely in plant and honey.
Allergenics: The product contains none of the ingredients of the list of allergenics of the EC.
APPLICATION:
It is widely used as nutrition, sweetener and process additive for food processing use and intravenous injection.
TECHNICAL SPECIFICATIONS:
|
Items |
Oral Grade Dextrose Monohydrate (GB/T20880-2007) |
Injection Grade Dextrose Monohydrate (BP2015) |
|
Appearance |
White granular powder |
White granular powder |
|
Odor and taste |
Smellless & sweet |
Smellless & sweet |
|
Dextrose content |
≥99.5% |
|
|
Specific optical rotation |
+52.0o-+53.5o |
+52.6o-+53.2o |
|
PH value |
4.0-6.5 |
|
|
Acidity |
≤1.2ml |
≤0.2ml |
|
Clarity of solution |
|
≤1# of turbidity standard |
|
Clarity of ethanol solution |
|
Clear |
|
Chloride |
≤0.02% |
≤0.01% |
|
Sulfate |
≤0.02% |
≤0.01% |
|
Insoluble matter in ethanol |
≤5mg |
|
|
Sulfite and soluble starch |
Meet the specification |
Meet the specification |
|
Loss on dry |
≤9.5% |
≤7.5-9.5% |
|
Ash |
≤0.6% |
|
|
Solubility |
≥99% |
|
|
Residue on ignition |
≤0.2% |
≤0.1% |
|
Protein |
|
Meet the specification |
|
Ferric salt |
≤0.002% |
≤0.001% |
|
Heavy metals |
≤20ppm |
≤5ppm |
|
Lead |
≤0.5ppm |
|
|
Arsenic salt |
≤0.0002% |
≤0.0001% |
|
Microbial limits |
Meet the specification |
Meet the specification |
|
Total plate count |
≤1500cfu/g |
|
|
Coliform Bacilli |
≤30MPN/100g |
|
|
Pathogenic Bacteria |
Negative |
|
|
Bacterial endotoxin |
|
≤0.25Eu/ml |
Net weight: 25kgs, gross weight: 25.3kgs, pp-woven bags.
20.0MT per 20’ container without pallet and 16.0MT per 20’ container with pallets.
STORAGE AND TRANSPORTATION:
The product should be stored on the pallet, in a light-proof, well-closed, cool, dry and ventilated place. Following the above guidelines should insure 24-month shelf life. After opening the original packing, the complete content should be used immediately. Material can be microbiologically contaminated from unhygienic environment. It is a non-dangerous product and can be transported as a common chemical product. Protect them from sunshine or rain. Must not be loaded and transported with toxic, hazard outstand polluting substances. Handle with care in order to avoid damaging the package.
QUALITY AND SAFETY ASSURANCE:
Controlled under certified quality system ISO and product safety is ensured by established safety system.
TEST METHOD: Full details and test method are available on request.
 Tel: +86-532-86990169|
Tel: +86-532-86990169| Email: sales@dailyfoodgroup.cn
Email: sales@dailyfoodgroup.cn

